HOW IS AI HELPING TO ADVANCE THERANOSTICS?
- Pharma Nuclear Services

- Oct 4, 2023
- 5 min read
June 28, 2023
In general terms, “theranostics” is the fusing of diagnosis and therapy to treat a variety of cancers, including prostate, breast, thyroid, bone, neuroendocrine, and others. Blended with artificial intelligence (AI), the field of theranostics may well be the next big shift in precision medicine—using patient-specific data to deliver precise and individualized diagnosis, treatment, and follow-up care. Here, Dr. Baris Turkbey, senior clinician in NCI’s Molecular Imaging Branch, Center for Cancer Research, explores the field of theranostics and describes how AI and data are helping shape a new generation of theranostics for cancer treatment.
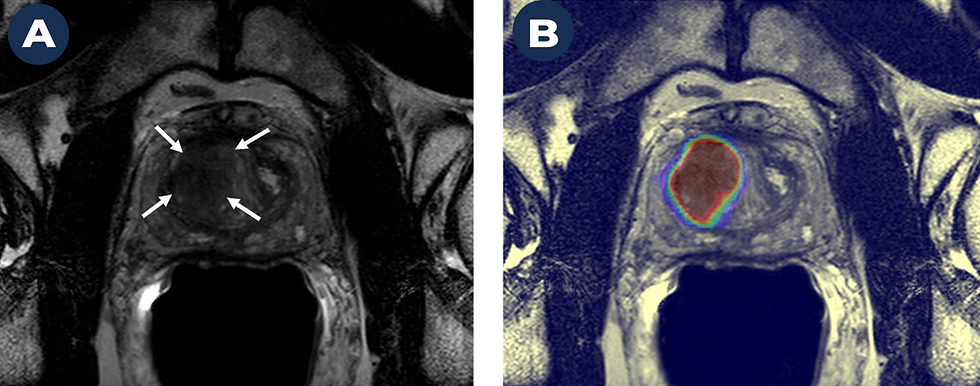
We evaluated a patient with a high PSA level (indicating a risk for prostate cancer) and a strong family history of prostate cancer. A Magnetic Resonance Image (MRI) on the left (A) shows a suspicious cancer lesion (marked by arrows). The image on the right (B) shows a high probability of prostate cancer within this lesion based on a deep learning-based AI algorithm. We biopsied the lesion using a technology that “fuses” transrectal ultrasoundwith MRI and were able to identify the early stages of clinically significant prostate cancer (Gleason 3+4).
What is “theranostics”?
Theranostics isn’t a new term. It’s been used in medicine for a while, but it’s definitely seeing more popularity now. It’s a concept in medicine that unites diagnostics with therapy.
Those therapeutic strategies might include chemotherapy, gene therapy, radiation, and more. We combine treatment approaches with diagnostic techniques, such as Positron Emission Tomography (PET), computed tomography (CT), and Magnetic Resonance Imaging (MRI), to develop molecular diagnostic tests and targeted therapeutics. This combination enables us to image, diagnose, and treat cancer, and allows us to monitor the response of that treatment—all at one time.
Theranostics has become more relevant recently because we have more precise techniques for identifying complex biological processes. Molecular imaging techniques, like PET, let us see areas of tumors that are most likely to respond to treatment, and we can select therapies that are most likely to work in those instances. The general concept in theranostics is, if you can image a certain biological entity, then you can more precisely treat that lesion or tumor with a targeted approach.
Can you give some examples of how theranostics are being applied to cancer research?
One example is prostate cancer, which our branch has been researching for more than 15 years. A molecule called prostate-specific membrane antigen (PSMA) is highly expressed in the majority of prostate cancers, making it a strong biomarker for disease. By linking a small radioactive particle (i.e., tracer) to this targeted biomarker (i.e., probe), we can image and treat cancer more precisely and monitor the effects of that treatment during follow-up care. The usefulness of this approach extends beyond prostate cancer. If you can molecularly profile a cancer, you can identify other abundantly expressed targets within that cancer that can serve as probes.
Another example of theranostics is the delivery of highly targeted therapies. My colleague, Dr. Frank Lin, is using Lutathera®, a radioactive drug that’s been specifically engineered to target and kill rare tumors such as pheochromocytoma and paraganglioma. We also can deliver focused therapy using near infrared photoimmunotherapy, which targets cancer cells with an antibody-photo absorber combination that causes the cells to swell and burst. This technique has shown promise in clinical trials for treating inoperable head and neck tumors.
These are some recent advances. Combining theranostics with AI is opening up even more areas of research.
How is AI helping to advance theranostics?
AI is a very popular term right now. It’s turning up everywhere these days. AI may not have all the answers yet, but it does help strengthen our traditional analysis methods. Take imaging for example. Whether we’re using CT, MRI, X-ray, or ultrasound, we miss quite a bit of information in these images. AI, which utilizes computer vision, can analyze these medical images in ways we never could before.
AI also helps us identify certain patterns. We can match those patterns to oncological outcomes, such as a histopathology result or overall survival, which is especially useful for making predictions early in the course of disease, when cancer is most treatable. Not only can we identify lesions or tumors using AI, we also can predict which sections of that tumor will result in poorer outcomes. This information potentially helps us target treatment and aids in identifying biomarkers.
Most importantly, AI offers an objective perspective. That objective approach can improve the treatment design process and increase our ability to predict which therapies will work best in each situation. We can deliver therapy to people with cancer using AI-assisted theranostics and, during follow up, AI can evaluate that treatment in a very objective way.
Ultimately, our human eye and human computation power have certain limits. AI helps us address these limits by connecting diagnostic findings with outcomes. We also can link imaging information with other genetic, molecular, clinical, and family-history data to find new biomarkers. AI depends on data. The more data we have, the more accurate and useful our AI will become.
You mention the value of using other types of data. What other data are needed or should be explored to further advance AI-theranostics?
We obtain a lot of data, such as lab data for example—blood, serum, urine. I’m not sure we’ve completely explored the value of all these data and how they can help our AI models.
For example, at NCI, we have a very strong AI model for prostate cancer. The model uses MRI to identify suspicious areas that could be cancerous. But this model assigns the same risk to a patient with elevated PSA as someone with prostatitis, which is an inflammatory disease. That’s because the imaging findings for cancer and prostatitis are very similar. When our predictions are based only on these imaging data, it can lead to false-positive results. We need additional clinical information to make the most accurate diagnosis.
This shows the value of having more multi-dimensional data. When we can combine clinical symptoms, family history, etc., with our MRI findings, we will have a much stronger model. This is a good example of how more data can help make AI more precise.
Theranostics has a lot of potential. How far away are we from having this technology in clinics?
AI depends heavily on data, and nuclear medicine is a very data rich field of medicine. This sets up the perfect situation for refining and moving this technology forward. Right now, a lot of people are investing time and resources, with very good collaborations between AI scientists and key societies, such as the Society of Nuclear Medicine and Molecular Imaging. With such a strong collaborative approach, it won’t take long, perhaps a few short years, before we begin to fully translate this technology into clinical practice.
Source :
https://datascience.cancer.gov/news-events/blog/theranostics-and-ai-next-advance-cancer-precision-medicine




Comments